
From January 29 to 30, 2024, a “randomized, double-blind, placebo-controlled Phase II/III clinical trial evaluating the safety and efficacy of Deuremidevir Hydrobromide dry suspension in hospitalized infants and children with Respiratory Syncytial Virus infection” was successfully initiated at Shulan (Hangzhou) Hospital, Linfen Central Hospital, and Mianyang Central Hospital. This signifies that Deuremidevir Hydrobromide (V116) dry suspension for the treatment of Respiratory Syncytial Virus (RSV) infection in infants and children has officially advanced to the Phase II clinical trial stage.
The study is a randomized, double-blind, placebo-controlled multicenter trial, aimed at evaluating the safety, efficacy, and pharmacokinetic characteristics of different doses of VV116 dry suspension in treating RSV infection in infants and children. Li Lanjuan, an academician of the Chinese Academy of Engineering and a renowned infectious disease scientist in China, serves as the leading principal investigator (Leading PI) of this research. The plan is to enroll 60 subjects, with participation from 20 research centers nationwide.
RSV, a single-stranded, negative-sense RNA virus, is capable of causing respiratory infections in individuals across all age groups. It is particularly impactful on infants, the elderly, and adults with immune deficiencies or underlying diseases, potentially leading to severe infections and respiratory sequelae. RSV is a major pathogen responsible for Acute Lower Respiratory Tract Infections (ALRTI) in children under 5 years old globally. Statistics indicate that RSV infection accounts for 28% of all ALRTI, and RSV-related hospital deaths account for 13%-22% of ALRTI- associated deaths. Currently, there are no globally approved specific treatments for RSV, highlighting a persistent unmet clinical need and a substantial disease burden.
VV116 is a novel oral nucleoside prodrug with independent intellectual property rights. It received conditional approval from the National Medical Products Administration in January 2023 for the treatment of mild to moderate COVID-19 in adult patients. VV116 exhibited broad-spectrum antiviral activities, showing strong inhibitory activity against various viruses such as coronaviruses (including SARS-CoV-2, OC43), respiratory syncytial virus, dengue fever virus, zika virus, ebola virus, etc.
VV116 demonstrates significant anti-RSV efficacy. It exhibited potent anti-RSV activities in A549, HEp-2, and NHBE cells (EC50 = 0.09~1.11 μM). The in vivo anti-RSV efficacy study of VV116 was conducted in 6–8 weeks old Balb/c mice. A twice-daily treatment for 4 days at an oral dose of 25 mg/kg VV116 decreased the infectious tilters by approximately 2 orders of magnitude; 50 mg/kg VV116 reduced the lung virus titers below the detection limit, and also alleviated lung injury caused by the virus.
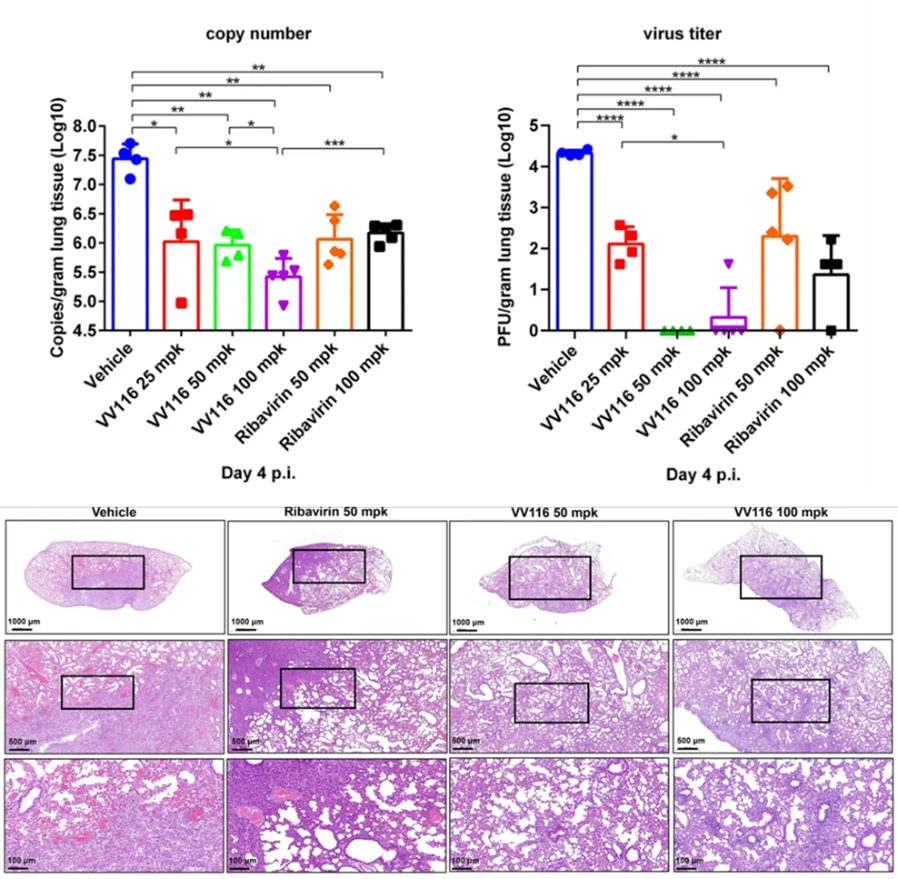
VV116 exhibits favorable pharmacokinetic properties. Phase I clinical study results show that a single oral dose of 25, 100, 300 mg of VV116 dry suspension, the main pharmacokinetic parameters of the main metabolite 116-N1 (parent nucleoside) increase proportionally, adhering to linear pharmacokinetic traits. The concurrent use of infant formula does not significantly impact the bioavailability of 116-N1.
VV116 has good safety profile. It exhibits no inhibitory impact on major drug metabolizing enzymes or transporters, and is devoid of genetic toxicity. The outcomes of a long-term (6 weeks) toxicity test in juvenile rats revealed that VV116 did not influence their growth and development, and no unforeseen toxicity was detected. The Phase I clinical findings demonstrated that the severity of all adverse drug reactions was ≤ 2, with no serious adverse events or adverse events ≥ 3 occurring. The incidence of adverse drug reactions did not display a clear dose-related trend, underscoring its overall safety.
We eagerly anticipate the swift progression of the Phase II/III clinical trials of VV116 dry suspension for the treatment of RSV infection in infants and children, and hope that it can will promptly introduce new treatment alternatives for patients.