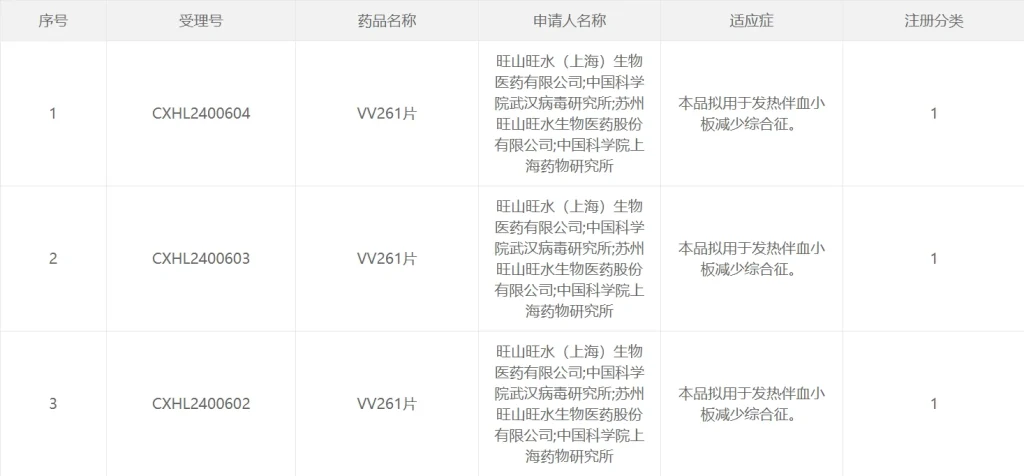
Vigonvita and Simcere Sign Exclusive Licensing and Collaboration Agreement for New Indications of VV116 On December 3, 2025, Vigonvita Life Science Co., Ltd. (2630.HK, hereinafter referred to as “Vigonvita”) entered into a licensing and collaboration agreement with Simcere Pharmaceutical Group (2096.HK, hereinafter referred to as “Simcere”). Under this agreement, Vigonvita will grant Simcere the exclusive rights to develop, manufacture, and commercialize VV116 for respiratory syncytial virus (RSV) infection and human metapneumovirus (HMPV) infection in Greater China (including the Chinese mainland, Hong Kong Special Administrative Region, Macao Special Administrative Region, and Taiwan region). Mr. Gaobo Zhou, Chief Investment Officer of Simcere, commented: “RSV affects people of all ages, and remains particularly harmful to infants, older adults, and individuals with weakened immune systems, for whom effective antiviral treatments are still limited. We are pleased to partner with Vigonvita to advance the development and commercialization of innovative antiviral therapies, with the hope of bringing more effective treatment options to patients as quickly as possible.” Dr. Guanghui Tian, Chairman and General Manager of Vigonvita, stated: “The dry suspension formulation of deuterated remididevir hydrobromide has received Breakthrough Therapy Designation and is expected to become the world’s first nucleoside-based antiviral approved for RSV. This exclusive licensing collaboration with Simcere will accelerate the development of indications for RSV and HMPV, with the goal…